

MicrobeCare : Independently, Clinically Studied
TM

Contact Us:
By Phone: +60 3 6413 2742
By Email: contact@microbeclean.asia
MicrobeCare has been clinically studied in the American Journal of Infection Control
by leading infection control epidemiologists and has shown persistence in preventing
the rebound effect of microorganisms.
“We found that a single application of MicrobeCare™, [provides]
a significant and persistent, long-term reduction in O.R. surface
contamination.”
Dr. Charles E. Edmiston Jr.
PhD, SM (ASCP), CIC (CBIC), FIDSA, FSHEA, FAPIC
Emeritus Professor of Surgery,
Medical College of Wisconsin
Dr. Edmiston began studying MicrobeCare™ as our largest skeptic.
With decades of infection control experience in terminally clean operating rooms, he
was eager to challenge the claims of MicrobeCare™.
To challenge the chemical in a clinical setting rather than a Good Lab (GLP) Practice
setting, he conducted a study at Froedtert Hospital at the Medical College of
Wisconsin in Milwaukee. Dr. Edmiston tested the bioburden of terminally cleaned
O.R. surfaces prior to treating with a single application of MicrobeCare™
Since then, Dr. Edmiston has independently studied MicrobeCare™ in the intensive
care unit (ICU) setting and is currently working on an emergency room study.
Published in the American Journal of Infection Control
Operating Room Study
4 operating rooms in a medical center were sampled three times over a 6-week
period. The operating rooms included two general surgical O.R., a hybrid O.R.
where open and endovascular procedures are performed, and an O.R. used for kidney
and liver transplants. The O.R.’s were used as normal during the 6-week study.
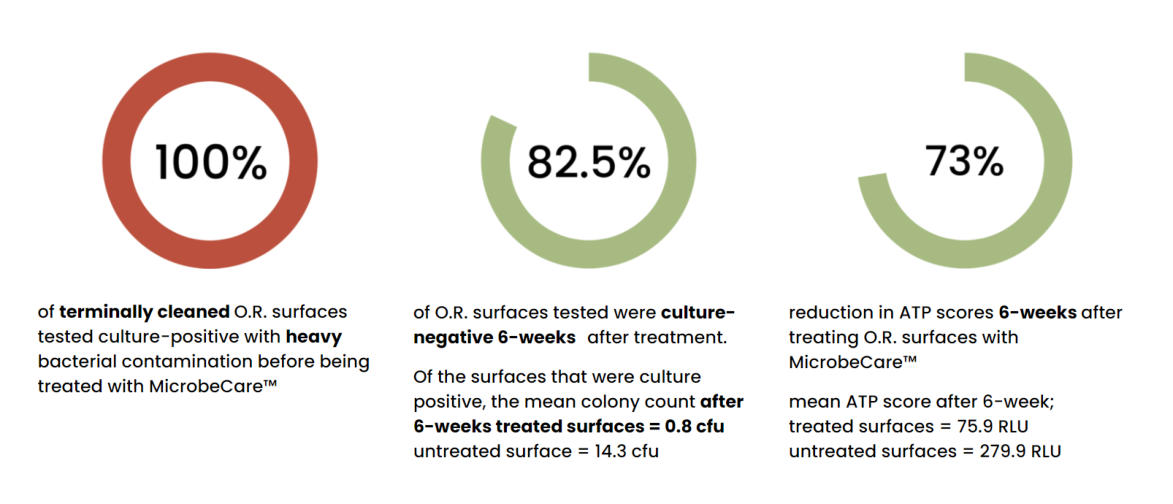
Operating Room Study
Selective high touch surfaces in a 26-bed medical intensive care unit at a tertiary
medical center were sampled over a 6-week period. The sample sites included
telephone handpieces, computer keypads, surfaces of physician workstations, and
selective patient items (blood pressure cuffs and patient bed tables).

Durability Testing of MicrobeCare: Phase 1
The test was set up to verify the effectiveness of MicrobeCare activated on plastic surfaces and then exposed
1200 Cleaning Cycles of Hospital Disinfectant, simulating 12 years of cleaning. Five different disinfectant that are
regularly used in hospitals were chosen to use when wiping the samples in the aging process.
Next, the samples were sent to NAMSA for Phase 2, further biological testing to ensure the maximum time the
antimicrobial will be effective.
To simulate up to 12 years of cleaning the surface of 5 plastic plates commonly used in operating rooms.
This test will verify the effectiveness of the MicrobeCare solution activated on plastic surfaces and then exposed
to various industrial cleaners over a period of time.
The samples went through an accelerated aging process that involved wiping the samples 100 to 1200 times with
five different cleaners that are regularly used in hospitals. This is equivalent to 12 years of cleaning at a hospital.
Next, the samples were sent to NAMSA for further biological testing to ensure the maximum time the
antimicrobial will be effective.
Durability Testing of MicrobeCare: Phase 2
Purpose: To test the efficacy of MicrobeCare against
an array of bacteria after undergoing a 12 year simulated
cleaning cycle.
Process: The plates were contaminated with several
microorganisms including E-coli and MRSA and then
tested 4 hours later for contamination.
Results: All 4 plates treated with MicrobeCare
showed >99.99% reduction in
contamination.

What microorganisms does MicrobeCare protect against?
MicrobeCare has been globally tested and shown to be effective against these specific types of organisms:
Bacteria
Micrococcus sp.
Mycobacterium smegmatis
Staphylococcus epidermidis 1
Mycobacterium tuberculosis (TBT)
Enterobacter agglomerans 1
Brucella cania
Acinetobacter calcoaceticus 1
Brucella abortus
Staphylococcus aureus (pigmented)
Brucella suis
Staphylococcus aureus
(non-pigmented) 1
Streptococcus mutans
Klebsiella pneumoniae ATCC 4352
Bacillus subtilis
Pseudomonas aeruginosa1
Bacillus cereus
Clostridium perfringens
Clostridium difficile (C.diff)
Psedomonas aeruginosa PDR-10
Haemophilus influenzae
Streptoccoccus faecelis
Haemophilus suis
Escherichis coli ATCC 23266
Lactobacillus casei
Escherichia coli1
Leuconostoc lactis
Proteus mirabilis 1
Listeria monocytogenes
Proteus mirabillisMicrococcus sp.
Propionibacterium acnes
Citrobacter diversus
Proteus vulgarisSalmonella typhosa
Pseudomonas cepacia
Salmonella choleraesuis
Pseudo Fluorescens
Corynebacterium Boris
Xanthomonas campestres
Methicillin Resistant
Staphylococcus aureus, (MRSA)
Vanconmycin Resistant enterococci (VRE)
Fungi
Fusarium solani
Penecillium humicola
Gliocladium roseum
Penicillium notatum
Oospora lactis
Penicillium variabile
Stachybotrys atra
ASpergillus niger
Mucor sp.
Aspergillus furmigatus
Tricophyton mentagrophytes
Aspergillus flavus
Trichoderma flavus
Aspergillus terreus
Chaetomium globusum
Penicillium chrysogenum
RRhizopus nigricans
Penicillium albicans
Cladosporium herbarum
Penicillium citrinum
Aureobasidium pullulans
Pencillium elegans
Fusarium nigrum
Penecillium funiculosu
Algae
Oscillatoria borneti LB 143
Schenedesmus quadricauda
Anabaena cylindrical B- 1446-lC
Gonium sp . LB 9c
Selenastrum gracile B-325
Volvox sp . LB 9
Pleurococcus sp . LB 11
Chlorella vulgarus
Yeast
Saccbaromyces cerevisiae
Candida albicans
Virus
Herpes Simplex Type 1 (HSV)
Poliovirus Type 2
Bacteriophage T2
Coronavirus
Other Independent Lab Test Results available upon request



TM













